Now Reading: Emergence Of Diverse Ligase Reactivities From A Single RNA Evolution Experiment
-
01
Emergence Of Diverse Ligase Reactivities From A Single RNA Evolution Experiment
Emergence Of Diverse Ligase Reactivities From A Single RNA Evolution Experiment
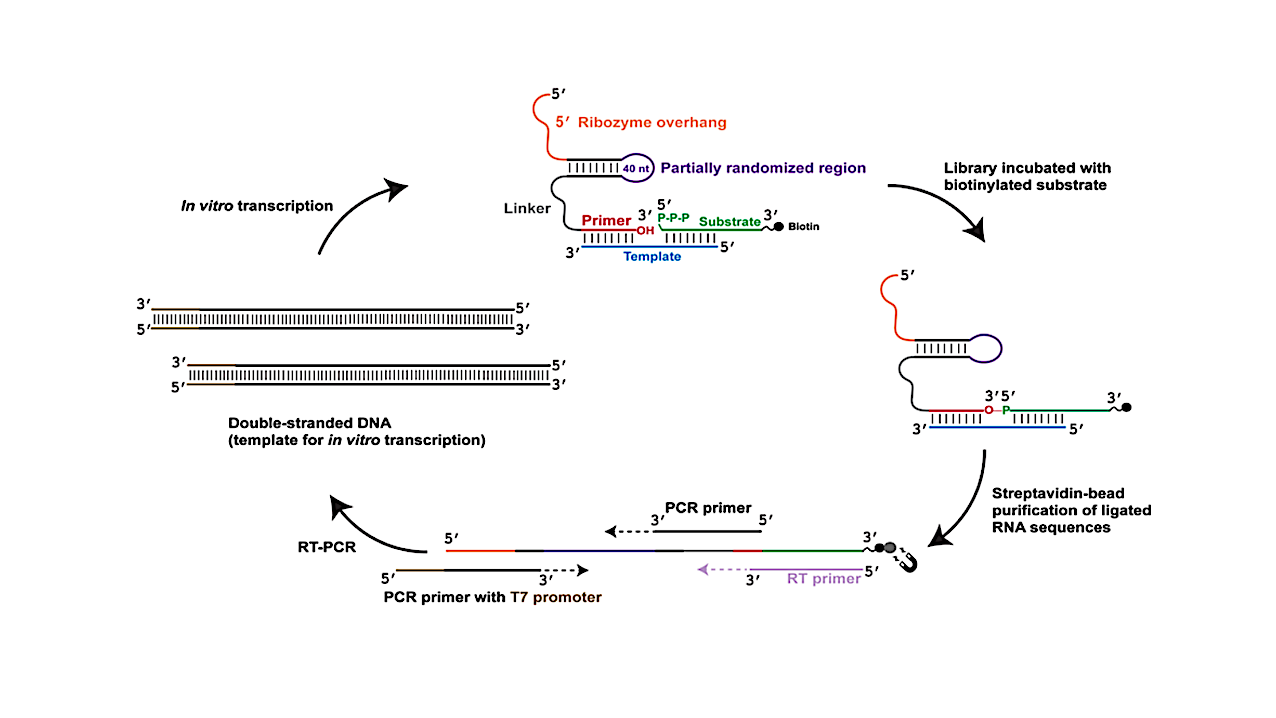

Selection protocol used to isolate ligase ribozymes. An RNA library containing a partially randomized sequence (depicted in purple) derived from an AIP-ligase18 was challenged with an RNA substrate (depicted in green), containing a 5′-triphosphate group and a 3′- TEG-biotin group (depicted in black) in the presence of an RNA template (depicted in blue). Ligated sequences were purified by binding to streptavidin-coated magnetic beads (depicted as a gray circle) and reverse transcribed using a primer (RT primer; depicted in light purple) that is complementary to the entire substrate sequence. The RT primer also has the potential to bind directly to the library sequences by forming four base-pairs with the 3′ end of the ‘primer’ (depicted in red). The cDNA was PCR-amplified, with the T7 promoter sequence (depicted in brown) added to the dsDNA sequence during PCR. This dsDNA was transcribed to generate the library for subsequent rounds of selection. — biorxiv.org
RNA-catalyzed assembly of RNA strands was one of the most essential enzymatic functions in a primordial RNA-based biology.
To explore the scope of RNA-catalyzed RNA assembly, we used directed evolution to re-engineer a ligase ribozyme that originally used 5′-phosphorimidazolide RNA substrates into a ribozyme that ligates RNA substrates carrying the biologically relevant 5′-triphosphate group.
Unexpectedly, analysis of the low-abundance regime of the selected RNA population, representing ∼0.1% of the population, revealed four distinct types of ligase ribozymes, indicating that multiple ribozymes had emerged from a single experiment, despite the stringent selection for the desired ‘triphosphate ligase’.
The first ligase type exhibits strict specificity for 5′-phosphorimidazolide substrates, even though these substrates were never presented during selection. The second and third ligase types catalyze two different branching reactions, each involving the 5′-triphosphate groups on the ribozyme and a different internal hydroxyl group on the substrate.
These reactions resemble ‘branching’ reactions catalyzed by naturally occurring ribozymes, including the spliceosome, even though these ribozymes emerged from a synthetic RNA library with no relation to biological RNA catalysts. The fourth ligase type mediates a reaction between its 5′-triphosphate and the substrate’s terminal 2′-OH, but only when the substrate carries a 3′-phosphate.
Remarkably, a single point mutation toggles this ribozyme between linear ligation and branching, functioning as a unique reactivity switch. Such a switch provides a mechanism for RNA catalysts to acquire new reactivities with minimal mutational perturbation, and therefore, has interesting evolutionary implications for primordial biocatalysis.
More generally, the emergence of such catalytic diversity from a single evolution experiment under highly constrained selection pressures highlights the catalytic flexibility and evolutionary potential of RNA. These findings strengthen the plausibility that an RNA World could have supported a wide range of chemistries essential for the emergence of life.
Emergence of diverse ligase reactivities from a single RNA evolution experiment, biorxiv.org
Astrobiology
Stay Informed With the Latest & Most Important News
Previous Post
Next Post
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors
03Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors -
 04Φsat-2 begins science phase for AI Earth images
04Φsat-2 begins science phase for AI Earth images -
 05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 06Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
06Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly -
 07Worlds Next Door: A Candidate Giant Planet Imaged in the Habitable Zone of α Cen A. I. Observations, Orbital and Physical Properties, and Exozodi Upper Limits
07Worlds Next Door: A Candidate Giant Planet Imaged in the Habitable Zone of α Cen A. I. Observations, Orbital and Physical Properties, and Exozodi Upper Limits