Now Reading: Chemistry in Extreme Environments: The Mystery of Molecular Complexity In Space
-
01
Chemistry in Extreme Environments: The Mystery of Molecular Complexity In Space
Chemistry in Extreme Environments: The Mystery of Molecular Complexity In Space

Chemistry in Extreme Environments — ACS Central Science
Molecular complexity in the interstellar medium (ISM) poses one of the most intriguing challenges in astrochemistry: how can chemical reactions operate efficiently under the extreme physical conditions of space?
In this Outlook, we summarize recent advances in understanding the molecular synthesis in the ISM, emphasizing the interplay between gas-phase and grain-surface chemistry. Laboratory studies, ranging from gas-phase kinetics at low temperature to the irradiation of interstellar ice analogues, demonstrate that both energetic and nonenergetic processes contribute to the formation of complex organic and prebiotic molecules.
We discuss how accurate exploration of reactive potential energy surfaces by means of quantum-chemical methodologies combined with kinetic simulations provide an atomistic interpretation of the interstellar processes.
Despite the advances of the past decade, interstellar chemistry remains in its infancy: reaction networks are incomplete, and quantitative predictions remain limited.

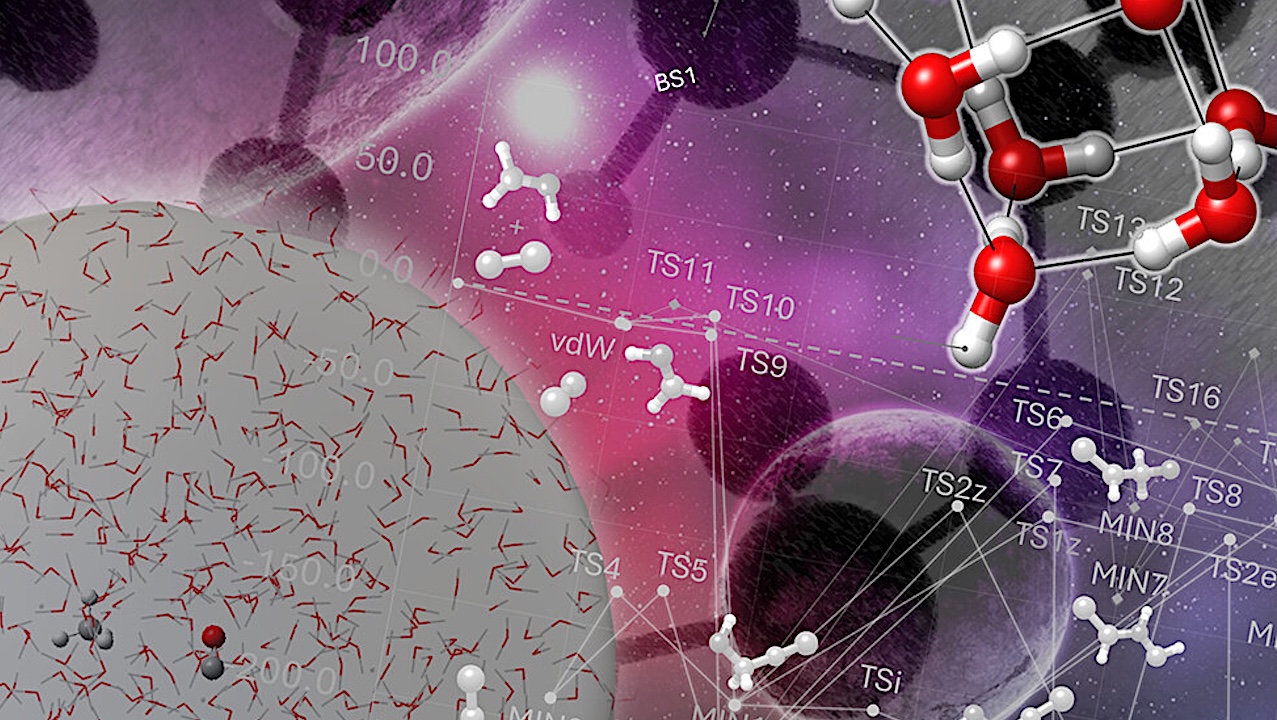
Panel (a) schematically illustrates the steps involved in the characterization of a generic reaction, HCN + XH2, occurring on the surface of interstellar ices. First, the gas-phase reaction is investigated to locate the corresponding TS for the association process. Then, an appropriate model is selected to describe the ice surface. Typically, the model is a cluster of water molecules or a cluster of CO species if an apolar ice is regarded. In the figure, a cluster of nine water molecules is chosen as an example. Depending on the adopted model, different binding sites (BSs) are available. The next step is the analysis of the binding energies (BEs) of the two isolated reactants adsorbed on the surface model. If two sites with large BEs are spatially close, it can be assumed that the reactants are in close proximity and react. Here, a different approach might be offered by the analysis of diffusion mechanisms of the reactants, which is however computationally more expensive. Using a cluster with the two reactants adsorbed as starting point, the TS is searched using that of the gas-phase reaction as a guidance. Then, the IRC analysis is employed to find the next intermediate of the reaction, which stabilizes if it lies lower in energy than the reactants. The intermediate might undergo further chemical processing, like hydrogenation or isomerization, or simply desorbes if the available energy exceeds its BE. Panel (b) shows a possible scheme for the reaction between CH4 and CO occurring inside the icy mantle of an interstellar grain. In this case, the cosmic rays penetrate the bulk and provide the energy for the reaction to take place in a concerted manner (with at least one species being activated by irradiation), thus leading to the final product (CH3CHO).– ACS Central Science via x-mol.net
Astrobiology, Astrochemistry,
Stay Informed With the Latest & Most Important News
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Φsat-2 begins science phase for AI Earth images
03Φsat-2 begins science phase for AI Earth images -
 04Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors
04Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors -
 05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 06Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
06Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly -
 07Worlds Next Door: A Candidate Giant Planet Imaged in the Habitable Zone of α Cen A. I. Observations, Orbital and Physical Properties, and Exozodi Upper Limits
07Worlds Next Door: A Candidate Giant Planet Imaged in the Habitable Zone of α Cen A. I. Observations, Orbital and Physical Properties, and Exozodi Upper Limits