Now Reading: A Close Unicellular Relative Reveals Aggregative Multicellularity Was Key To The Evolution Of Animals
-
01
A Close Unicellular Relative Reveals Aggregative Multicellularity Was Key To The Evolution Of Animals
A Close Unicellular Relative Reveals Aggregative Multicellularity Was Key To The Evolution Of Animals
Key animal multicellularity genes are expressed during M. vibrans aggregation. — biorxiv.org
How animals evolved complex multicellularity from their unicellular ancestors remains unanswered.
Unicellular relatives of animals exhibit simple multicellularity through clonal division, formation of multinucleate coenocytes, or aggregation1 Therefore, animal multicellularity may have evolved from one (or a combination) of these behaviours. Aggregation has classically been dismissed as a means to complex multicellularity.
However, aggregation occurs in many extant animal cells and has also been recently described in three different unicellular relatives of animals (the choanoflagellates Salpingoeca rosetta and Choanoeca flexa, and the filasterean Capsaspora owczarzaki). It is unclear whether aggregation in these species is derived or ancestral, and its relevance for animal origins remains unknown.
To fill this gap, we investigated whether an additional unicellular relative of animals can undergo aggregation. We discovered that the marine free-living bacterivorous filasterean Ministeria vibrans forms homogeneous aggregates with reproducible kinetics that have long-term stability when cultured with an alphaproteobacterium. We found that many multicellularity genes involved in animal cell adhesion, signaling, and transcriptional regulation were deployed during this process.
Our findings suggest that the last unicellular ancestor of animals had the capacity to aggregate using key animal multicellularity genes and that improved feeding and sexual reproduction may be evolutionary drivers of this aggregation.

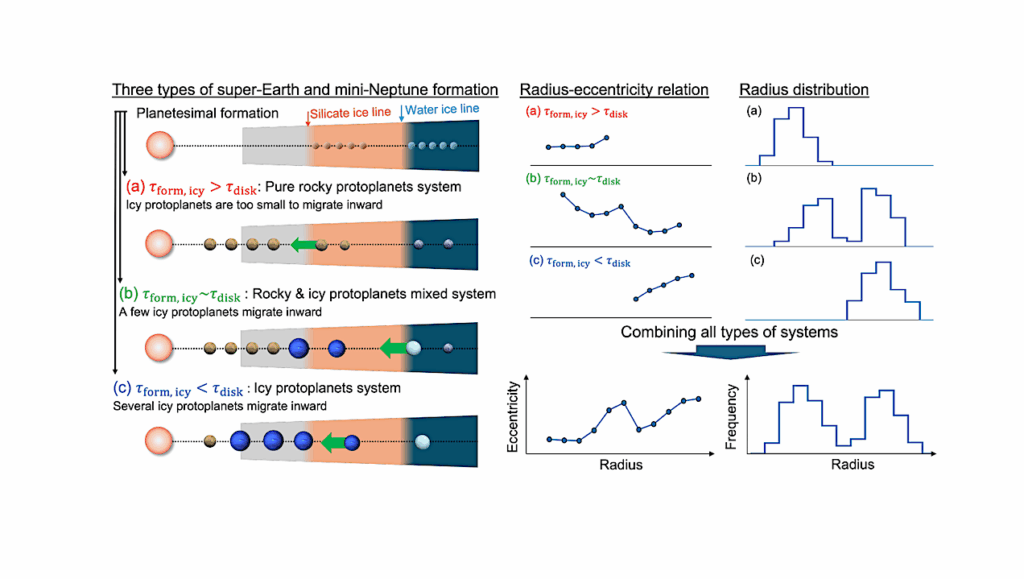
Multicellular aggregation in unicellular holozoans. a) Schematic phylogeny of holozoans. Orange circle represents the ancestor of Filozoa–the clade that comprises metazoans, choanoflagellates, and filastereans. b) The choanoflagellate S. rosetta forms moderately sized, stringy, multicellular aggregates in the presence of bacterial chondroitinases. 3 A fraction of C. flexa choanoflagellate cells also aggregate upon changes in salinity. 4 c) The filasterean C. owczarzaki homogeneously forms large multicellular aggregates in the presence of lipoproteins and phosphatidylcholine lipids from animal hosts. 5,6 Neither aggregative process has been monitored transcriptionally or proteomically across the entire range of development to determine which animal multicellularity genes are essential for the initial formation and maturation of aggregates in filozoans. — biorxiv.org
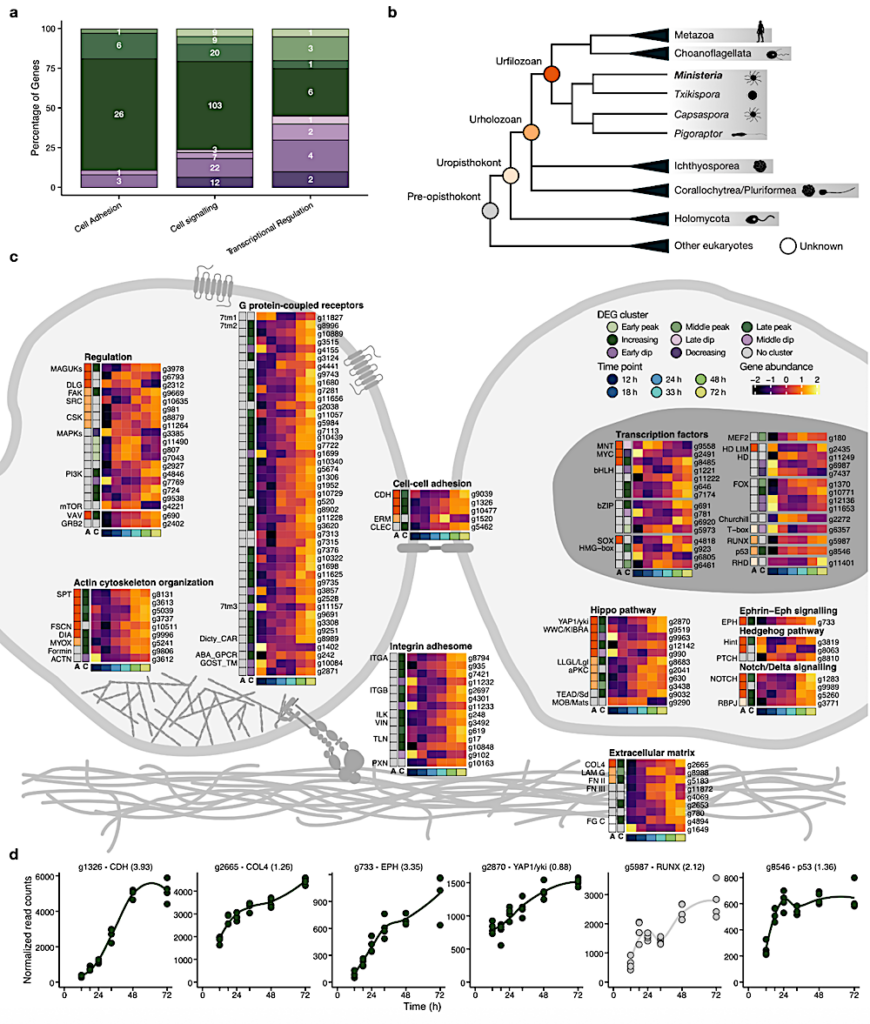
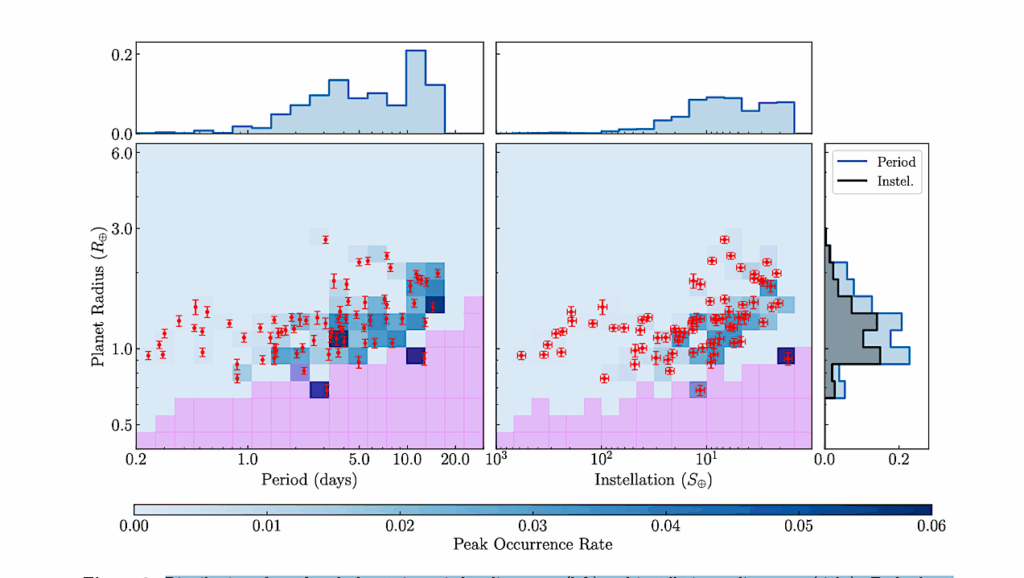
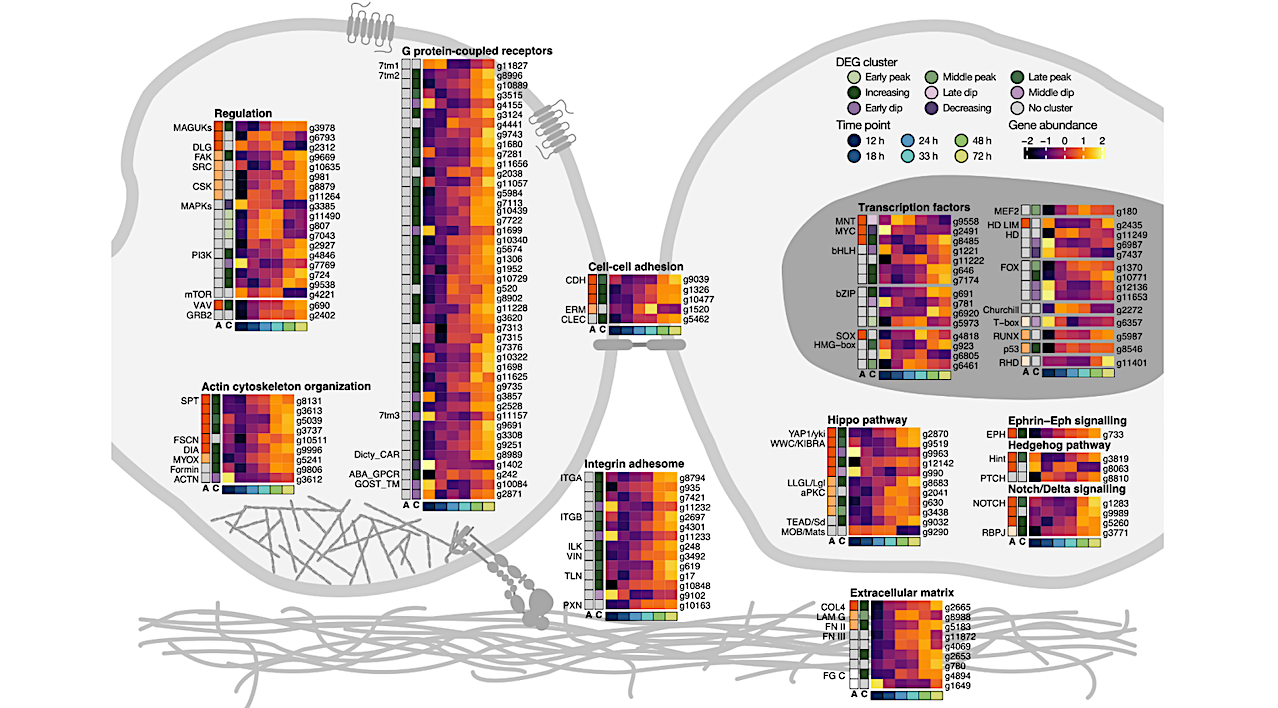
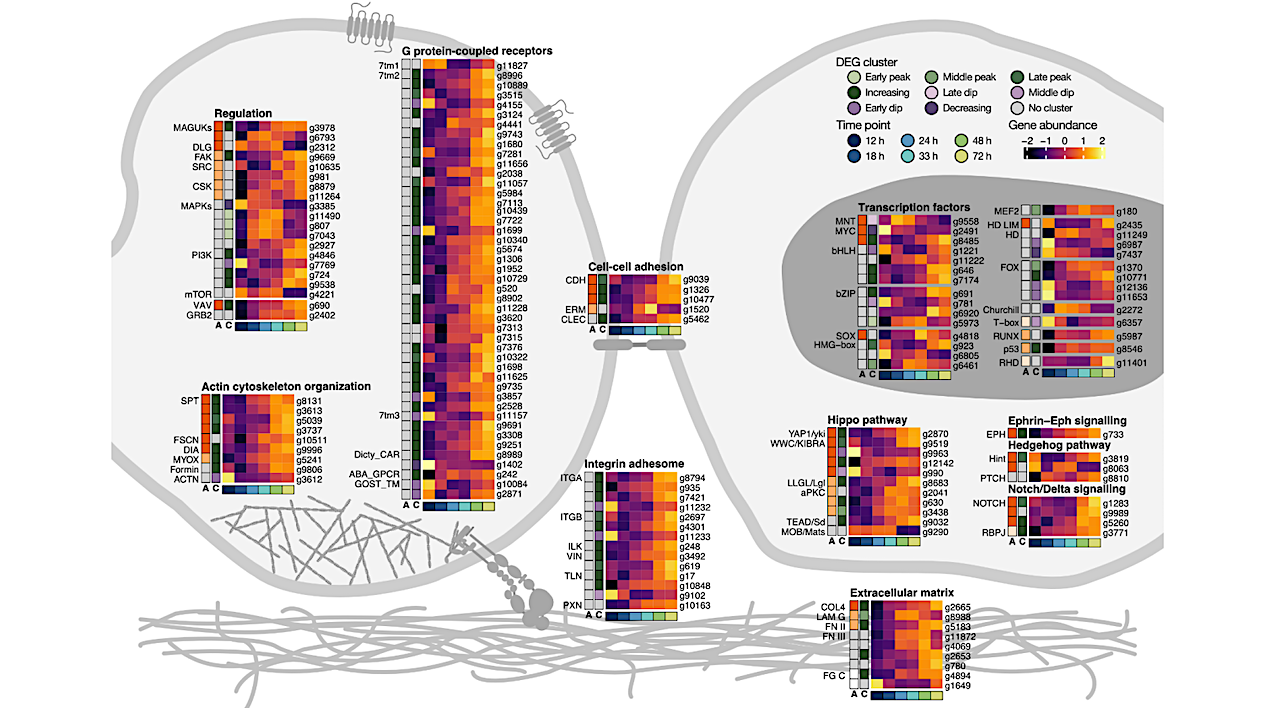
Key animal multicellularity genes are expressed during M. vibrans aggregation. a) Percentage of genes linked to cell adhesion, cell signalling, and transcriptional regulation assigned to each differentially expressed gene (DEG) cluster. b) Overview of Opisthokonta species relationships and ancestors indicated by coloured circles. c) Schematic of two M. vibrans cells and heatmaps showing the expression patterns of different categories of multicellularity-related genes that are significantly differentially expressed across the aggregation time series.
The bottom row indicates the time point according to the legend, the first column (column “A”) indicates the predicted gene age according to the coloured ancestors in panel b, and the second column (column “C”) indicates DEG cluster assignment. Mean gene abundances were calculated across time point replicates and centered and scaled using the variance-stabilizing transformation of normalized gene counts. Spectrin; SPT, fascin; FSCN, diaphanous; DIA, myosin X; MYOX, formin-like protein; Formin, alpha actinin; ACTN, alpha type IV collagen; COL4, laminin G; LAM G, fibronectin type II and type III; FN II and III, fibrinogen C-terminal; FG C, integrin alpha; ITGA, integrin beta; ITGB, integrin-linked kinase; ILK, alpha-catenin/vinculin family; VIN, talin; TLN, paxillin; PXN, cadherin; CDH, ezrin-radixin-moesin family; ERM, C-type lectin; CLEC, membrane-associated guanylate kinases; MAGUKs, discs large MAGUK; DLG, focal adhesion kinase; FAK, tyrosine-protein kinase c-Src; SRC, Cterminal Src kinase; CSK, mitogen-activated protein kinases; MAPKs, phosphatidylinositol 3 kinase; PI3K, mechanistic target of rapamycin; mTOR, Vav guanine nucleotide exchange factor; VAV, growth factor receptor-bound protein 2; GRB2, rhodopsin/class A; 7tm1, adhesion/secretin/class B; 7tm2, glutamate/class C; 7tm3, cAMP/classE; Dicty_CAR, abscicic acid receptor; ABA_GPCR, GOST seven transmembrane; GOST_TM, yes-associated protein 1/yorkie; YAP1/yki, WW and C2 domain containing/kidney and brain expressed protein; WWC/KIBRA, lethal 2 giant larvae; LLGL/Lgl, protein kinase C; aPKC, TEA domain transcription factor/scalloped; TEAD/Sd, MOB kinase activator/mob as tumor suppressor; MOB/Mats, eph receptor; EPH, Hint/Hog domain found in Hedgehog; Hint, patched; PTCH, notch; NOTCH, recombining binding protein suppressor of hairless; RBPJ, basic helix loop helix; bHLH, MAX network transcriptional repressor bHLH; MNT, MYC proto-oncogene bHLH; MYC, basic leucine zipper; bZIP, high mobility group box; HMG-box, SRY-related HMG-box; SOX, myocyte-specific enhancer factor 2 MADS-box; MEF2, homeodomain; HD, forkhead; FOX, runt-related; RUNX, rel homology DNA-binding; RHD. d) Plots of normalized gene counts across the aggregation time series for selected upregulated genes with diverse functions and filozoan or holozoan origins. Log2 fold change between 12 and 72 h is indicated in brackets. See also Extended Data Fig. 9, Fig. S3, and Data S10. — biorxiv.org
A close unicellular relative reveals aggregative multicellularity was key to the evolution of animals, biorxiv.org
Astrobiology, evolution,
Stay Informed With the Latest & Most Important News
Previous Post
Next Post
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04True Anomaly hires former York Space executive as chief operating officer
04True Anomaly hires former York Space executive as chief operating officer -
 05Φsat-2 begins science phase for AI Earth images
05Φsat-2 begins science phase for AI Earth images -
 06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly