Now Reading: Atomistic Modeling of Methyl Formate and Glycolaldehyde Formation on Interstellar Dirty Ice Mantles via a “Radical + Ice” Mechanism
-
01
Atomistic Modeling of Methyl Formate and Glycolaldehyde Formation on Interstellar Dirty Ice Mantles via a “Radical + Ice” Mechanism
Atomistic Modeling of Methyl Formate and Glycolaldehyde Formation on Interstellar Dirty Ice Mantles via a “Radical + Ice” Mechanism
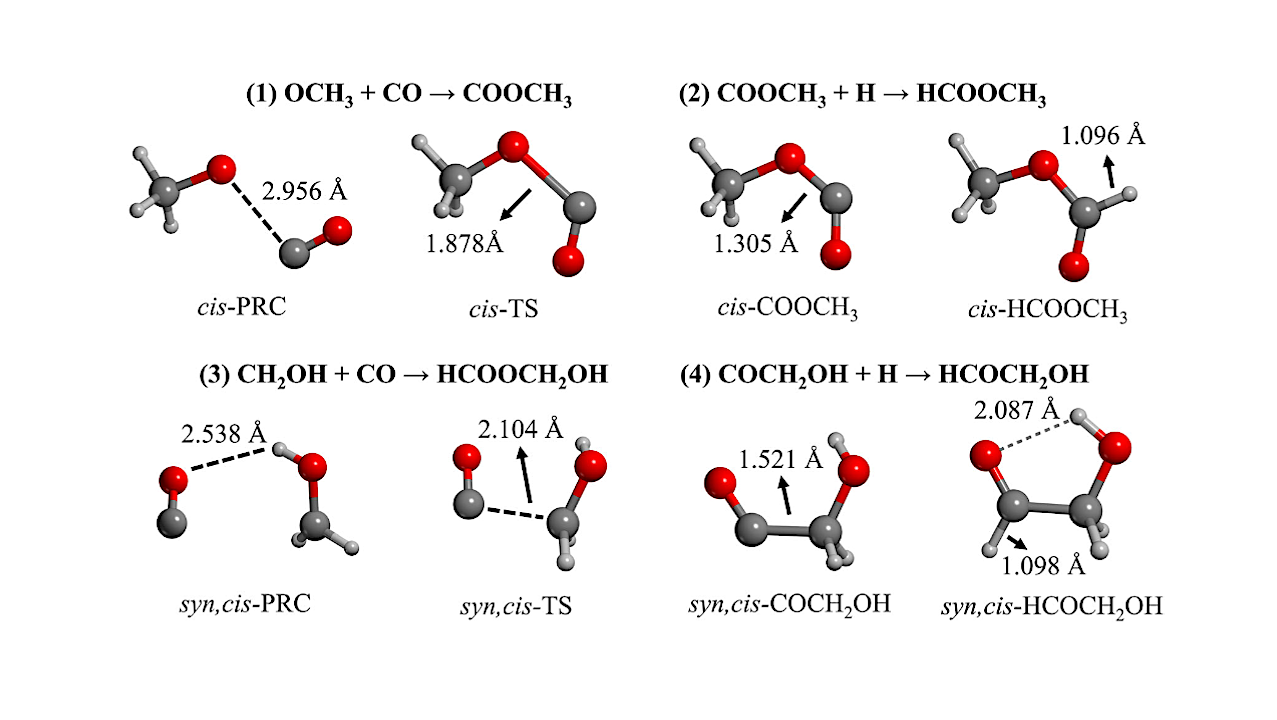

Stationary points of Equation (1) and (2) yielding the most stable cis-MF conformer, computed at MPWB1K-D3(BJ)/6-31G(d, p) theory level, and Equation (3) and (4) yielding the most stable syn, cis-GA conformer, computed at BHLYP-D3(BJ)/6-31G(d, p) level of theory. PRC stands for prereactant complex and TS for transition state. Distances are given in Å. The energetics can be found in Table 1. — Chempluschem
Methylformate (MF) and glycolaldehyde (GA) are two primogenital organic molecules detected in both cold and warm regions of the interstellar medium (ISM).
Both gas-phase and grain-surface pathways have been proposed to explain their abundances, yet uncertainties remain, since prevailing grain-surface mechanisms favor the formation of GA over MF, which mismatch observations in different ISM regions.
In this work, MF and GA synthetic reactions are atomistically modeled on surfaces containing variable H2O and CO percentages (interstellar dirty ices), in which one of the reactants coming from the gas phase reacts with an icy CO, thus adopting the following two-step “radical + ice” mechanism: for MF, OCH3 + CO(ice) → COOCH3 + H → HCOOCH3 ; for GA, CH2OH + CO(ice) → COCH2OH + H → HCOCH2 OH. Calculations show that the first step presents an energy barrier (32-38 kJ mol−1 for MF and 17-20 kJ mol−1 for GA), while the second step is nearly barrierless.
Although the energetics favor GA formation, the observed abundances are better explained by desorption phenomena rather than reaction barriers are argued. Specifically, the weaker binding energies of MF (16.8-46.1 kJ mol−1 ) than GA (28.4-90.2 kJ mol−1 ) support its higher abundance in the ISM.
Atomistic Modeling of Methyl Formate and Glycolaldehyde Formation on Interstellar Dirty Ice Mantles via a “Radical + Ice” Mechanism, PubMed via Chempluschem
Atomistic Modeling of Methyl Formate and Glycolaldehyde Formation on Interstellar Dirty Ice Mantles via a “Radical + Ice” Mechanism, Chempluschem (open access)
Astrobiology, Astrochemistry
Stay Informed With the Latest & Most Important News
Previous Post
Next Post
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04Φsat-2 begins science phase for AI Earth images
04Φsat-2 begins science phase for AI Earth images -
 05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 06True Anomaly hires former York Space executive as chief operating officer
06True Anomaly hires former York Space executive as chief operating officer -
 07Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors
07Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors