Now Reading: Computational Insights Into the Chemical Reaction Networks of C3H6O3,C3H7O3 and C2H5O2: Implications for the Interstellar Medium
-
01
Computational Insights Into the Chemical Reaction Networks of C3H6O3,C3H7O3 and C2H5O2: Implications for the Interstellar Medium
Computational Insights Into the Chemical Reaction Networks of C3H6O3,C3H7O3 and C2H5O2: Implications for the Interstellar Medium
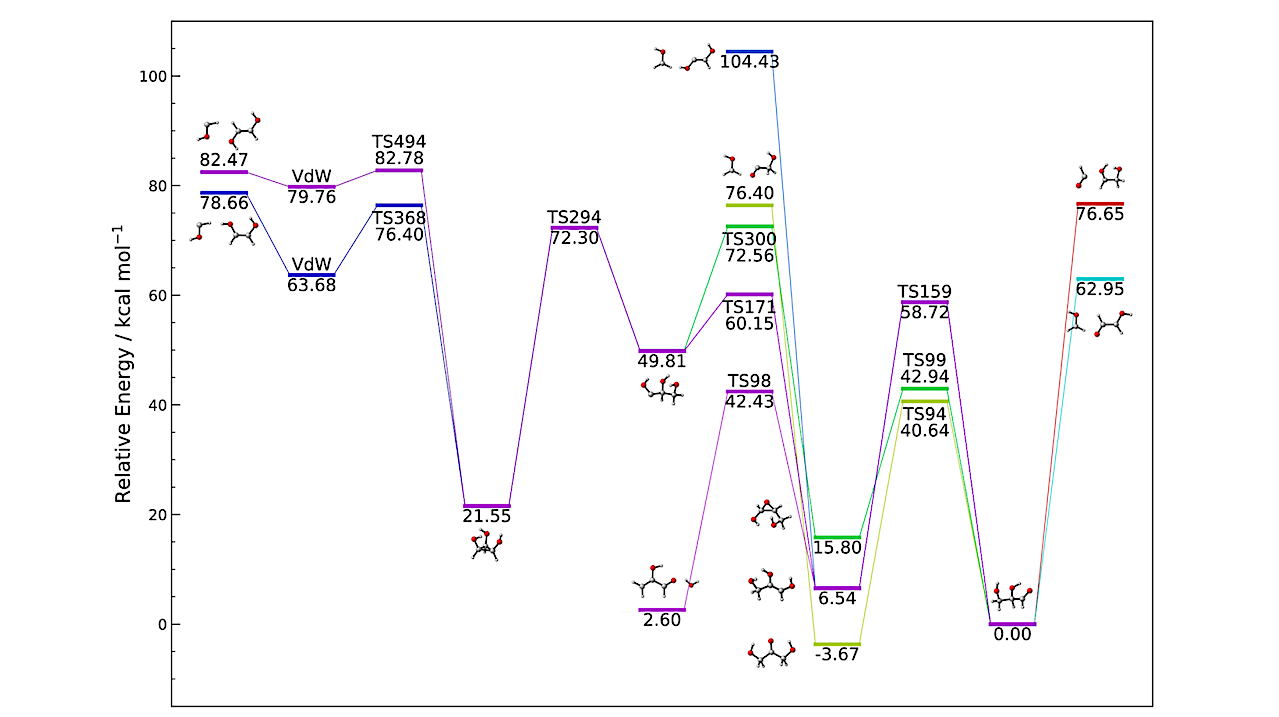

DFT-computed energy profile for the most probable formation routes of GCA via radiative association. The energies include zero-point vibrational energy (ZPE), with the zero of energy set for GCA, shown at the bottom right of the figure. — astro-ph.GA
The formation of complex organic molecules in the interstellar medium (ISM) is central to astrochemistry and prebiotic chemistry, as these species may act as precursors to biomolecules essential for life.
Although glyceraldehyde (HOCH2CH(OH)C(O)H, GCA) has not yet been detected in the ISM, the presence of structurally related compounds in various astronomical environments suggests that it may form under interstellar conditions.
In this study, we employed the automated reaction discovery tool AutoMeKin to systematically explore the gas-phase chemical reaction networks (CRNs) of C3H6O3 (GCA), C3H7O3 (a hydrogenated analog), and C2H5O2. Reaction pathways were characterized at the wB97XD/Def2-TZVPP level of theory, and rate coefficients for key processes were computed using the competitive canonical unified statistical (CCUS) model, which accounts for multiple dynamic bottlenecks.
Our analysis revealed several barrierless pathways leading to GCA or to GCA and a leaving group. Notably, the reaction between glyoxal (HCOHCO) and the HOCHCH2OH radical, though neither has yet been detected in the ISM, was found to efficiently produce GCA and a formyl radical.
However, aside from the aforementined exception, most GCA formation channels result in highly vibrationally excited intermediates that are more likely to undergo rapid unimolecular decomposition than to be stabilized by radiative emission under typical ISM conditions.
These results suggest that while gas-phase GCA formation is chemically feasible, it is likely transient and difficult to detect directly. In contrast, alternative products such as formaldehyde, glycolaldehyde, and (Z)-ethene-1,2-diol dominate many pathways and align better with current astronomical observations.
Anxo Lema-Saavedra, Antonio Fernández-Ramos, Emilio Martínez-Núñez
Subjects: Astrophysics of Galaxies (astro-ph.GA); Chemical Physics (physics.chem-ph)
Cite as: arXiv:2505.02427 [astro-ph.GA] (or arXiv:2505.02427v1 [astro-ph.GA] for this version)
https://doi.org/10.48550/arXiv.2505.02427
Focus to learn more
Submission history
From: Emilio Martinez-Nunez
[v1] Mon, 5 May 2025 07:46:34 UTC (46,156 KB)
https://arxiv.org/abs/2505.02427
Astrobiology,
Stay Informed With the Latest & Most Important News
Previous Post
Next Post
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04Φsat-2 begins science phase for AI Earth images
04Φsat-2 begins science phase for AI Earth images -
 05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 06Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors
06Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors -
 07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly