Now Reading: Electron Configuration And Catalytic Logic: A Physicochemical Framework For The Inorganic Origins Of Life
-
01
Electron Configuration And Catalytic Logic: A Physicochemical Framework For The Inorganic Origins Of Life
Electron Configuration And Catalytic Logic: A Physicochemical Framework For The Inorganic Origins Of Life
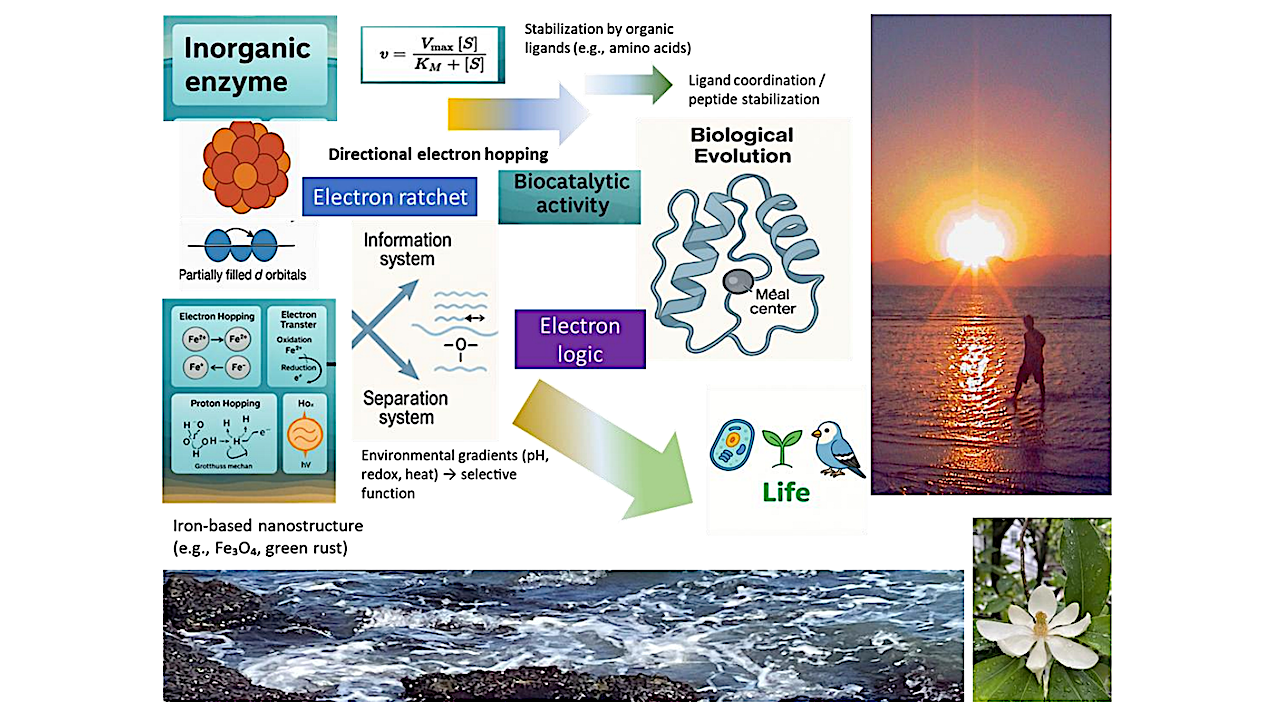

Graphical Abstract — chemrxiv.org
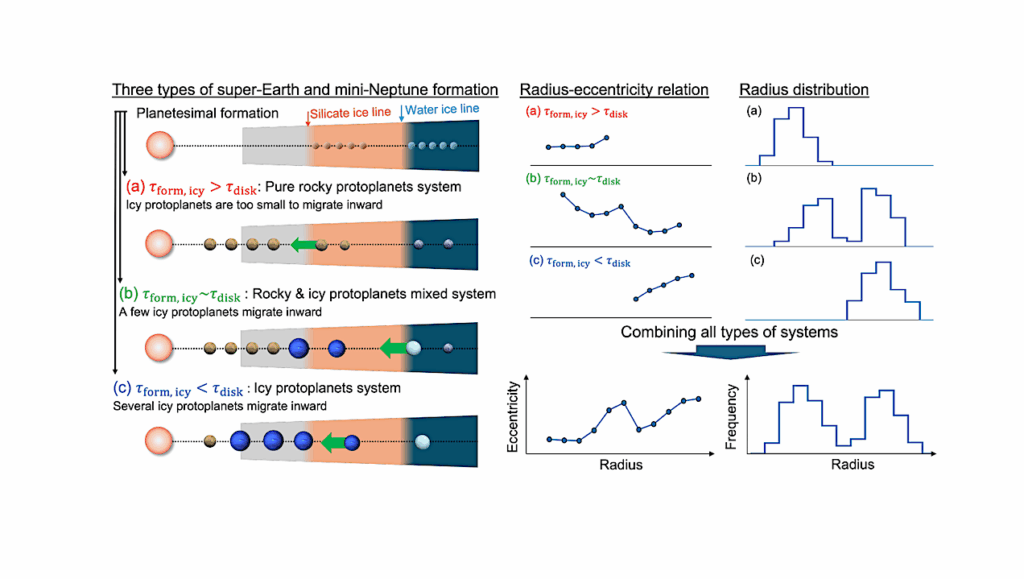
We present a physicochemical framework for understanding the emergence of catalytic logic at the origin of life. In this view, early catalytic systems did not depend on genetic encoding or biological membranes, but arose from the intrinsic properties of inorganic nanomaterials—particularly iron-based oxides and sulfides.
These materials, through their electron configurations and redox-active surfaces, supported charge hopping, intervalence transfer, and redox cycling. We argue that these processes enabled a directional flow of electrons, forming what we describe as an “electron ratchet”: a structural and energetic asymmetry that sustained catalytic cycles far from equilibrium.
Such solid-state catalysis, governed by spatial and temporal separation, offers a plausible prebiotic alternative to enzyme function. Small molecules may have stabilized these systems, leading to the progressive evolution of organic ligands and protein scaffolds that preserved and refined inorganic reactivity.
This perspective integrates mineral surface chemistry, nanostructured electron transfer, and systems-level principles to propose a continuous evolutionary pathway from rock to enzyme. We support this hypothesis with structural and mechanistic comparisons between modern metalloenzymes and their putative inorganic ancestors, highlighting redox geometries, charge flow dynamics, and catalytic selectivity.
The resulting framework suggests that metabolic logic was not invented, but inherited—from the physical and energetic rules of mineral catalysis. Life, in this view, emerges as an organized ratcheting of charge through constrained matter.
Electron Configuration and Catalytic Logic: A Physicochemical Framework for the Inorganic Origins of Life, chemrxiv.org
Astrobiology,
Stay Informed With the Latest & Most Important News
Previous Post
Next Post
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04True Anomaly hires former York Space executive as chief operating officer
04True Anomaly hires former York Space executive as chief operating officer -
 05Φsat-2 begins science phase for AI Earth images
05Φsat-2 begins science phase for AI Earth images -
 06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly