Now Reading: From Amino Acids to α-Keto Acids via β-Elimination and Transamination Initiates a Pathway to Prebiotic Reaction Networks
-
01
From Amino Acids to α-Keto Acids via β-Elimination and Transamination Initiates a Pathway to Prebiotic Reaction Networks
From Amino Acids to α-Keto Acids via β-Elimination and Transamination Initiates a Pathway to Prebiotic Reaction Networks
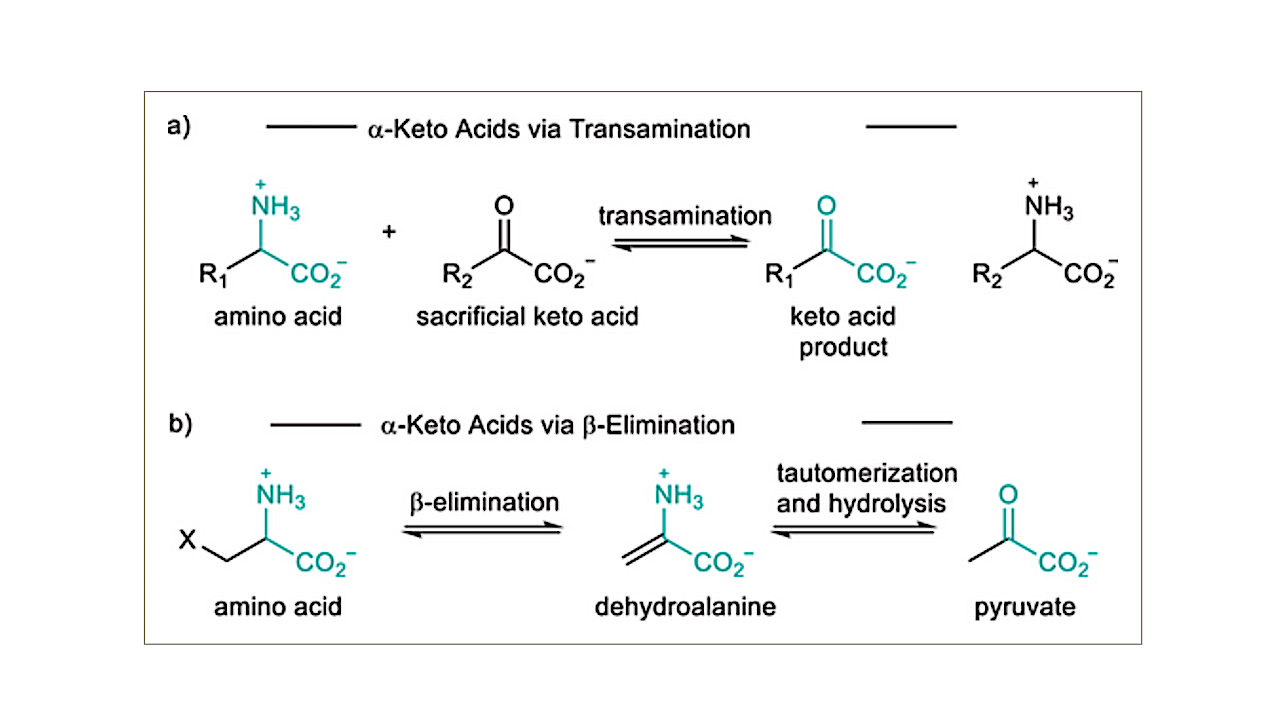

Routes to α-keto acids from amino acids. a) The conversion of an α-amino acid to an α-keto acid via transamination requires an additional α-keto acid; however, b) the β-elimination of a side-chain activated amino acid produces an α-keto acid (pyruvate) directly. — Angewandte Chemie International Edition
α-Keto acids, such as pyruvate and glyoxylate, may have been critical in generating reaction networks at the origins of life due to their facile carbon-carbon bond formation and their hydrolytic stability.
However, demonstrated prebiotic sources of these small α-keto acids have been limited by conditions required for their production, which are not conducive for subsequent incorporation or transition into (proto)metabolic pathways.
Here, we demonstrate an abiotic generation of α-keto acids from only two amino acids, starting with the phosphorylation and dehydration of serine (Ser) coupled with a transamination with glycine (Gly), to produce both pyruvate and glyoxylate. This triggers an in situ reaction pathway producing higher-order α-keto acids, including amino acid precursors found in modern biology.
These findings may help elucidate how protometabolic chemical networks can emerge on the early Earth under mild aqueous conditions, leading to a coupled amino acid-α-keto acid chemical system capable of supporting a more robust metabolism.

The phosphorylation and β-elimination of Ser, coupled with a transamination with Gly. This series of reactions yields the two simplest α-keto acids, pyruvate, and glyoxylate which undergo aldol and Cannizzaro chemistry initiating a reaction sequence generating higher order α-keto acids, including TCA cycle intermediates. — Angewandte Chemie International Edition via PubMed
From Amino Acids to α-Keto Acids via β-Elimination and Transamination Initiates a Pathway to Prebiotic Reaction Networks, Angewandte Chemie International Edition via PubMed (open access)
Astrobiology, Astrochemistry,
Stay Informed With the Latest & Most Important News
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04True Anomaly hires former York Space executive as chief operating officer
04True Anomaly hires former York Space executive as chief operating officer -
 05Φsat-2 begins science phase for AI Earth images
05Φsat-2 begins science phase for AI Earth images -
 06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly