Now Reading: Interstellar Chemistry of CN Radicals on Ices: The Formation of CH3CN and CH3NC and Potential Connection to Acetamide
-
01
Interstellar Chemistry of CN Radicals on Ices: The Formation of CH3CN and CH3NC and Potential Connection to Acetamide
Interstellar Chemistry of CN Radicals on Ices: The Formation of CH3CN and CH3NC and Potential Connection to Acetamide
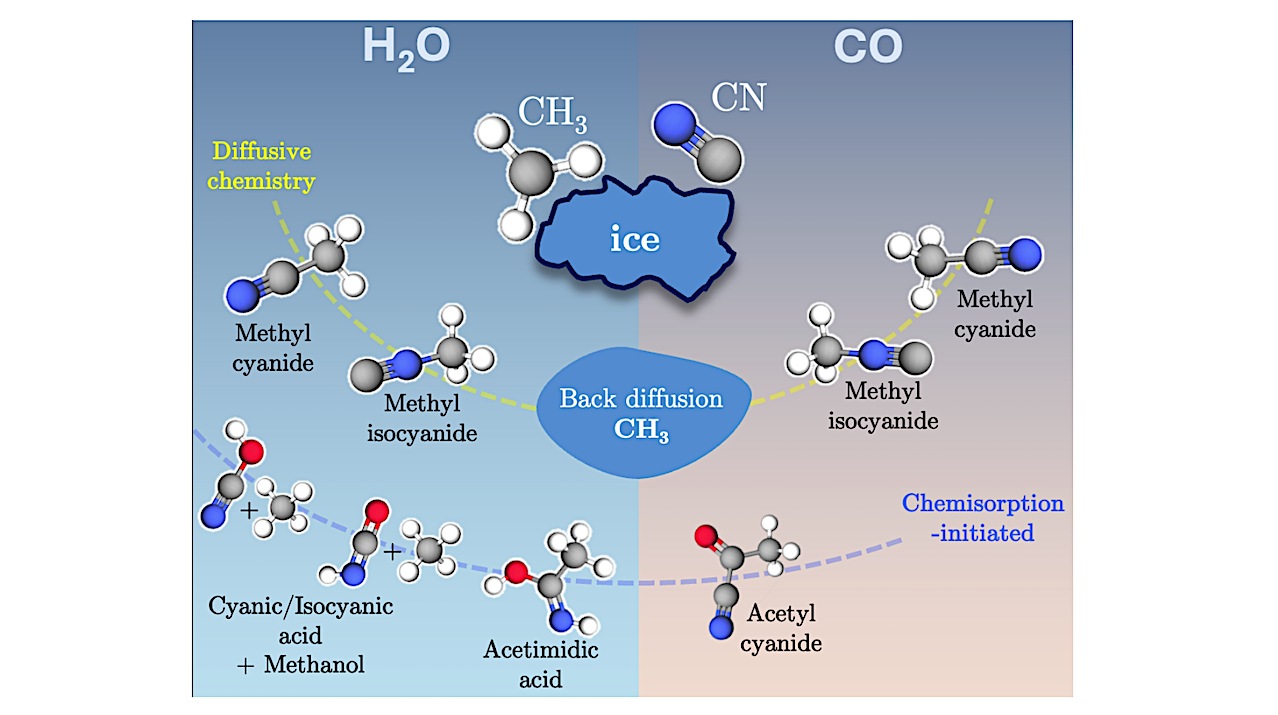

Summary of the reactions studied in this work between •CH3 + •CN on water and carbon monoxide ice surfaces. The first line indicates the possible outcomes of diffusive chemistry, while the second indicates the products after the reaction with one of the ice molecules from the surface. Notice in the chemisorption initiated products can proceed from two different mechanisms: chemisorption followed by Habstraction (cyanic and isocyanic acid) or chemisorption followed by radical-radical coupling (acetimidic acid and acetyl cyanide). — astro-ph.GA
Context. Among the most significant chemical functional groups of interstellar molecules are the class of nitriles, which are proposed as key prebiotic molecules due to their chemical connection to the peptide bond after hydrolysis. CN radicals, the simplest representative of this group, have been shown to exhibit strong interactions with interstellar water ices, potentially impacting their reactivity with other radicals nearby.
Aims. This study explores (a) whether CN and CH3 radicals can readily react to form methyl cyanide (CH3CN) and its isomer methyl isocyanide (CH3NC); and (b) the feasibility of the reaction (CN…H2O)hemi -> C(OH)=NH and its potential role in the formation of acetamide. Methods. Following a benchmark, density functional theory was employed to map the potential energy surfaces of these chemical processes, focusing on their reactivity on water and carbon monoxide ices.
Results. The results show that CN reacts with CH3 radicals on water ices, forming CH3CN and CH3NC efficiently. However, these reactions are driven by diffusion of CH3 towards the reactive site and subsequently compete with back-diffusion of CH3 from that site. The formation of the radical intermediate C(OH)=NH on water ice requires quantum tunnelling and assuming that acetimidic acid forms via CH3 + C(OH)=NH -> CH3C(OH)=NH, it can also only isomerize into acetamide through a sizable barrier thanks to quantum tunnelling. Both quantum tunnelling-driven reactions are highly dependent on the local structure of the water ice. Finally, radical coupling reactions on carbon monoxide ices are found to be barrierless for all cases and again, both the cyanide and the isocyanide are formed.
Conclusions. This work reinforces the conclusion that CN radicals on interstellar grain surfaces are highly reactive and unlikely to persist unaltered.
Joan Enrique-Romero, Thanja Lamberts
Comments: Accepted in A&A, June 2025
Subjects: Astrophysics of Galaxies (astro-ph.GA)
Cite as: arXiv:2506.08792 [astro-ph.GA] (or arXiv:2506.08792v1 [astro-ph.GA] for this version)
https://doi.org/10.48550/arXiv.2506.08792
Focus to learn more
Submission history
From: Joan Enrique-Romero
[v1] Tue, 10 Jun 2025 13:41:13 UTC (8,211 KB)
https://arxiv.org/abs/2506.08792
Astrobiology, Astrochemistry,
Stay Informed With the Latest & Most Important News
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04True Anomaly hires former York Space executive as chief operating officer
04True Anomaly hires former York Space executive as chief operating officer -
 05Φsat-2 begins science phase for AI Earth images
05Φsat-2 begins science phase for AI Earth images -
 06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly