Now Reading: Is Ozone a Reliable Proxy for Molecular Oxygen? II. The impact of N2O on the O2-O3 relationship for Earth-like atmospheres
-
01
Is Ozone a Reliable Proxy for Molecular Oxygen? II. The impact of N2O on the O2-O3 relationship for Earth-like atmospheres
Is Ozone a Reliable Proxy for Molecular Oxygen? II. The impact of N2O on the O2-O3 relationship for Earth-like atmospheres
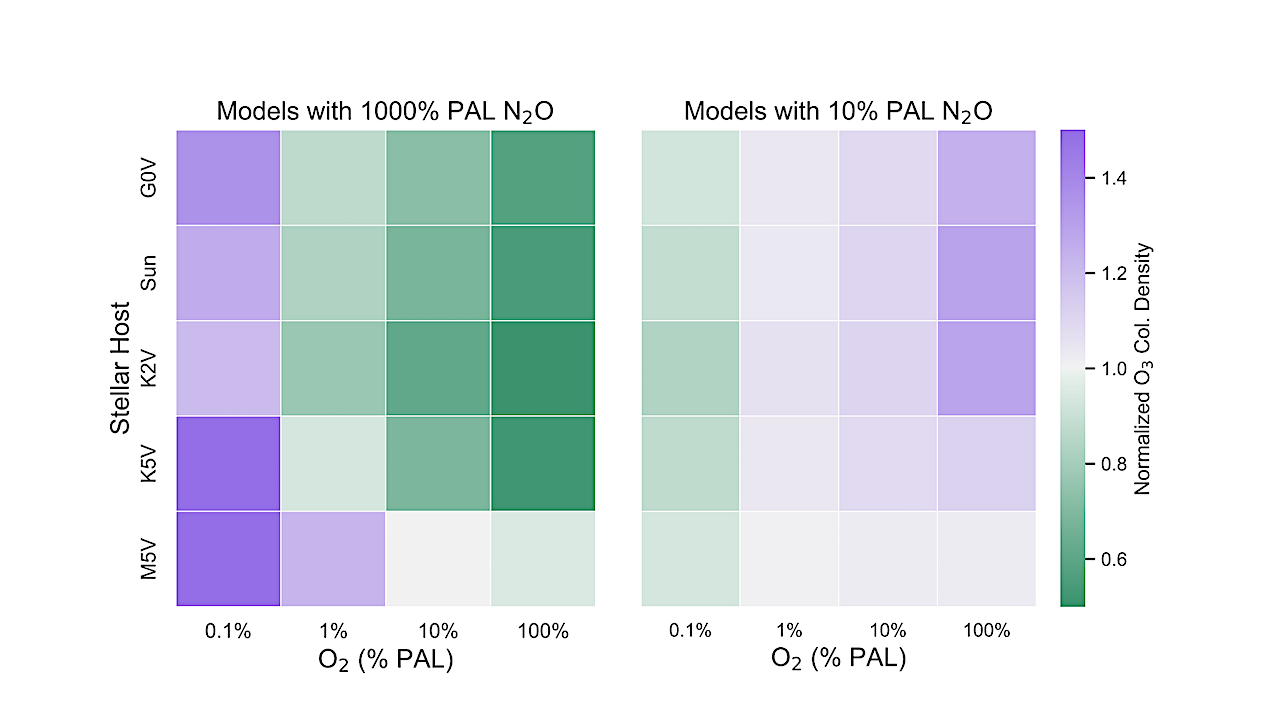

Total O3 abundances for models with high N2O (left) and low N2O (right) normalized to models with modern levels of N2O from Kozakis et al. (2022). Overall the high N2O models impacted the O2-O3 relationship more than the low N2O models, with results being highly dependent on the stellar host and the amount of O2. High N2O models with hotter stars experience significant O3 depletion due to faster NOx catalytic cycles caused by increased N2O. However, for the high N2O models at very low O2 levels planets around all hosts experience an increase in O3 caused by the higher efficiency of the smog mechanism once the Chapman mechanism is limited by low amounts of O2. The M5V-hosted planet in particular experiences an increase of O3 with the high N2O models starting at 10% PAL O2 and lower due to the increased capabilities of the smog mechanism in this lower UV environment. — astro-ph.EP
Molecular oxygen (O2) will be an important molecule in the search for biosignatures in terrestrial planetary atmospheres in the coming decades.
In particular, O2 combined with a reducing gas is thought to be strong evidence for disequilibrium caused by surface life.
However, there are circumstances where it would be very difficult or impossible to detect O2, in which cases it has been suggested that ozone (O3), the photochemical product of O2, could be used instead. Unfortunately, the O2-O3 relationship is highly nonlinear and dependent on the host star, as shown in detail in the first paper in this series.
We explore the O2-O3 relationship around G0V-M5V host stars, using climate/photochemistry modeling to simulate atmospheres while varying abundances of O2 and nitrous oxide (N2O). N2O is of particular importance to the O2-O3 relationship not just because it is produced biologically, but because it is the primary source of nitrogen oxides (NOx), which fuel the NOx catalytic cycle which destroys O3, and the smog mechanism that produces O3. We vary the O2 mixing ratio from 0.01-150% present atmospheric level (PAL), and N2O abundances of 10% and 1000% PAL.
We find that varying N2O impacts the O2-O3 relationship differently depending strongly on both the host star and the amount of atmospheric O2. Planets orbiting hotter hosts with strong UV fluxes efficiently convert N2O into NOx, often depleting a significant amount of O3 via faster NOx catalytic cycles. However, for cooler hosts and low O2 levels we find that increasing N2O can lead to an increase of overall O3 due to the smog mechanism producing O3 in the lower atmosphere.
Variations in O3 result in significant changes in the amount of harmful UV reaching the surfaces of the model planets as well as the strength of the 9.6 μm O3 emission spectral feature, demonstrating potential impacts on habitability and future observations.
Thea Kozakis, João M. Mendonça, Lars A. Buchhave, Luisa M. Lara
Comments: Accepted to A & A
Subjects: Earth and Planetary Astrophysics (astro-ph.EP)
Cite as: arXiv:2505.23279 [astro-ph.EP](or arXiv:2505.23279v1 [astro-ph.EP] for this version)
https://doi.org/10.48550/arXiv.2505.23279
Focus to learn more
Submission history
From: Thea Kozakis
[v1] Thu, 29 May 2025 09:25:30 UTC (440 KB)
https://arxiv.org/abs/2505.23279
Astrobiology,
Stay Informed With the Latest & Most Important News
Previous Post
Next Post
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04Φsat-2 begins science phase for AI Earth images
04Φsat-2 begins science phase for AI Earth images -
 05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 06Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors
06Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors -
 07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly