Now Reading: Microbial Exoenzymes Catalyzed The Transition To An Oxygenated Earth
-
01
Microbial Exoenzymes Catalyzed The Transition To An Oxygenated Earth
Microbial Exoenzymes Catalyzed The Transition To An Oxygenated Earth
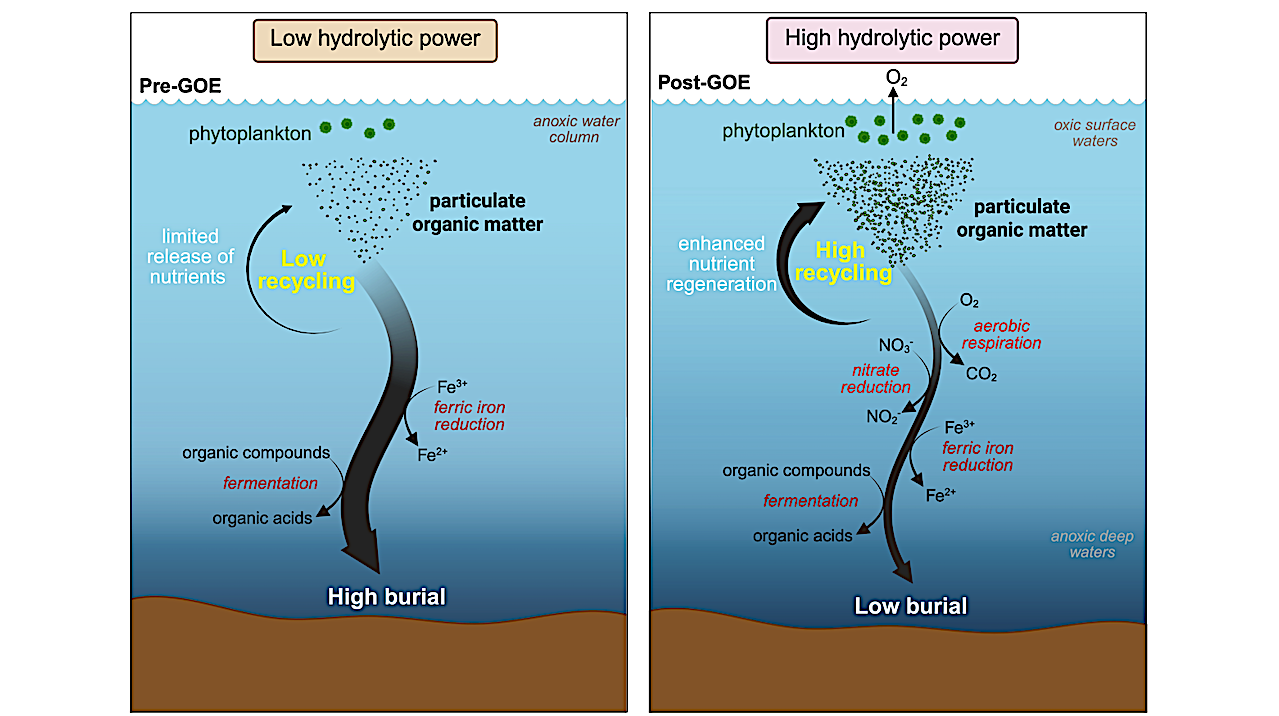

Conceptual model for the evolution of exoenzymes and the recycling of organic matter across the Great Oxidation Event (GOE). (A) In the Archean Ocean, prior to the GOE, the recycling of organic matter depended entirely on the activity of fermenters. This resulted in low recycling efficiency, elevated carbon burial, and limited regeneration of essential nutrients back into seawater. This scarcity of particulate organic matter (POM) also reduced the availability of niches for microbial colonization, thus limiting the selective pressure for the evolution of exoenzyme systems. (b) Following the GOE, rising oxygen levels increased the availability of high-energy electron acceptors (e.g. O2, nitrate and ferric iron), supporting the expansion of metabolic groups with high exoenzyme capacity, such as aerobic heterotrophs, dissimilatory nitrate reducers and dissimilatory ferric iron reducers. Concurrently, increases in POM abundance and microbial cell densities would have amplified the selective pressure to produce exoenzymes. Collectively, these changes in the Paleoproterozoic promoted more efficient recycling of organic matter and regeneration of nutrients back into the water column, ultimately fueling greater primary productivity and oxygen release. This feedback between exoenzyme evolution, nutrient cycling, and oxygen production would have amplified Earth’s progressive oxygenation. — biorxiv.org
Microbial exoenzymes—extracellular enzymes secreted to degrade complex organic polymers— are essential for recycling carbon and nutrients, thus sustaining primary productivity in today’s oceans.
Yet, their evolutionary history and role in shaping Earth’s early biosphere remain entirely unexplored.
Here, we trace the origins of microbial exoenzymes and reveal their previously unrecognized role in driving planetary oxygenation. Our results show that exoenzymes are more common in microorganisms utilizing high-energy metabolisms, likely reflecting the energetic costs of enzyme biosynthesis and secretion.
They are especially advantageous in environments rich in particulate organic matter (POM). A refined carbon cycle model indicates that early Archean oceans offered few such habitats, as low productivity and intense UV radiation rapidly photodegraded POM.
However, with a Paleoproterozoic rise of atmospheric oxygen, increased oxidative weathering boosted marine primary productivity and POM accumulation, creating conditions favoring exoenzyme evolution. Molecular clock analyses further indicate that alkaline phosphatase, a key phosphorus-releasing exoenzyme, had likely emerged with the permanent rise of oxygen, enabling more efficient phosphorus recycling.
We propose that exoenzymes initiated a positive feedback loop: by accelerating nutrient regeneration, they fueled cyanobacterial productivity and oxygen release, which in turn favored greater exoenzyme capacity, reinforcing long-term oxygenation of the planet.

Diverse metabolisms share functionally and genetically similar exoenzymes. Sequence similarity network of predicted exoenzymes associated with (A) enzyme type based on sequence annotation; and (B) metabolic guild. The 1307 exoenzyme protein sequences are depicted by individual nodes (colored circles). Edges (grey lines) between the nodes indicate a pairwise BLAST e-value of 1×10-30 345 . Each exoenzyme sequence is represented by a node (colored circle); 1,267 nodes are grouped into 48 clusters (e-value of 1×10-30 346 ) based on high sequence similarity, though 40 sequences were dissimilar from all other sequences and are thus not connected to any other nodes. Featured clusters (right panels) depict how diverse metabolic guilds share functionally and genetically similar exoenzymes. — biorxiv.org
Microbial exoenzymes catalyzed the transition to an oxygenated Earth, biorxiv.org
Astrobiology, genomics, evolution,
Stay Informed With the Latest & Most Important News
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04True Anomaly hires former York Space executive as chief operating officer
04True Anomaly hires former York Space executive as chief operating officer -
 05Φsat-2 begins science phase for AI Earth images
05Φsat-2 begins science phase for AI Earth images -
 06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly