Now Reading: Reasons Why Life on Earth Rarely Makes Fluorine-containing Compounds And Their Implications for the Search for Life Beyond Earth
-
01
Reasons Why Life on Earth Rarely Makes Fluorine-containing Compounds And Their Implications for the Search for Life Beyond Earth
Reasons Why Life on Earth Rarely Makes Fluorine-containing Compounds And Their Implications for the Search for Life Beyond Earth


Fluorine on Earth — Astrobiology.com
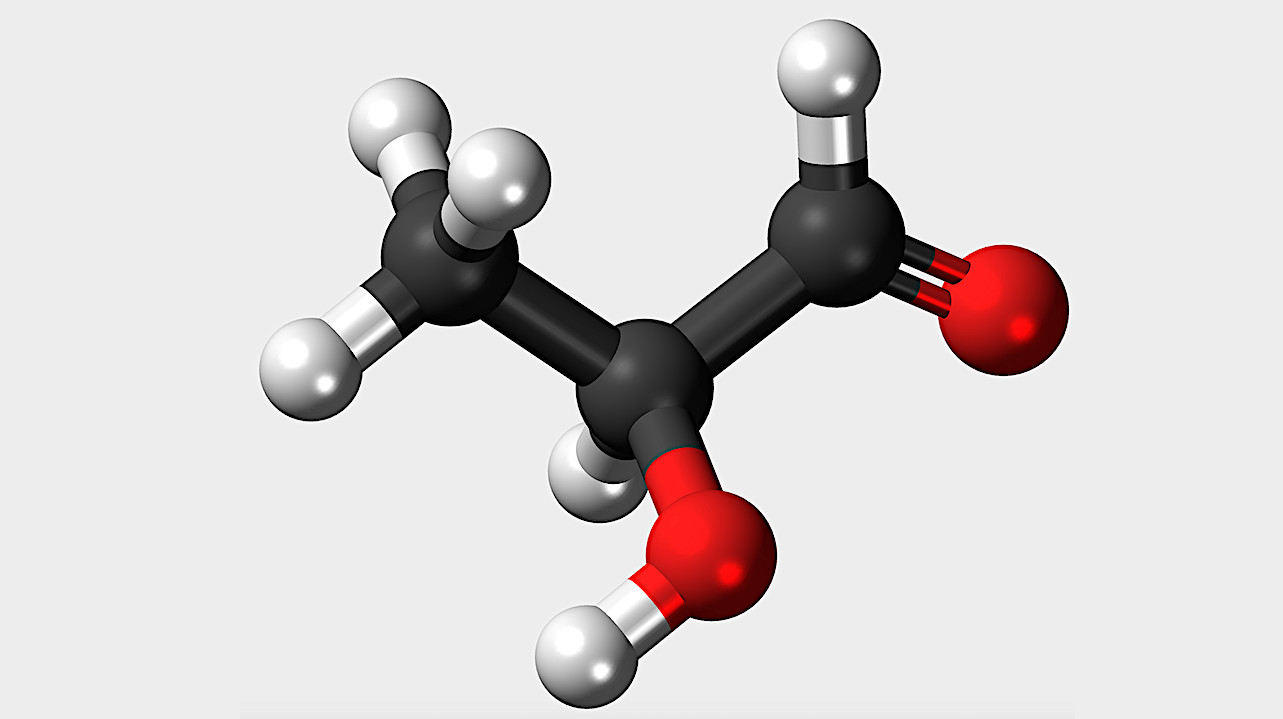
Life on Earth is known to rarely make fluorinated carbon compounds, as compared to other halocarbons. We quantify this rarity, based on our exhaustive natural products database curated from available literature.
We build on explanations for the scarcity of fluorine chemistry in life on Earth, namely that the exclusion of the C–F bond stems from the unique physico-chemical properties of fluorine, predominantly its extreme electronegativity and strong hydration shell.
We further show that the C–F bond is very hard to synthesize and when it is made by life its potential biological functions can be readily provided by alternative functional groups that are much less costly to incorporate into existing biochemistry.
As a result, the overall evolutionary cost-to-benefit balance of incorporation of the C–F bond into the chemical repertoire of life is not favorable. We argue that the limitations of organofluorine chemistry are likely universal in that they do not exclusively apply to specifics of Earth’s biochemistry. C–F bonds, therefore, will be rare in life beyond Earth no matter its chemical makeup.

Elemental abundances amongst natural products (NPs). The number of natural products in our database5 containing a given element and the percentage number are shown. The halogen atoms Cl- and Br-containing compounds are nearly as common as S–containing compounds amongst natural products. In contrary to Cl, Br and even I halogens, F-containing natural compounds are severely underrepresented in the chemical repertoire of life on Earth. The figure compilation shows only elements that can form covalent bonds that are stable in water. The compilation excludes transition metals from the analysis. (*) No molecules containing Si bonded to any atom other than oxygen are known to be made by life, although silica and silicic acid are used extensively by life on Earth8. — Science Reports via PubMed
Reasons why life on Earth rarely makes fluorine-containing compounds and their implications for the search for life beyond Earth, Science Reports via PubMed (open access)
Astrobiology, Astrochemistry, Biochemistry,
Stay Informed With the Latest & Most Important News
Previous Post
Next Post
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04True Anomaly hires former York Space executive as chief operating officer
04True Anomaly hires former York Space executive as chief operating officer -
 05Φsat-2 begins science phase for AI Earth images
05Φsat-2 begins science phase for AI Earth images -
 06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly