Now Reading: Revision of the Structure of Scytonemin Imine and Its Relationship to Scytonemin: Chromism and Implications for Astrobiology and Cyanobacterial Adaptability
-
01
Revision of the Structure of Scytonemin Imine and Its Relationship to Scytonemin: Chromism and Implications for Astrobiology and Cyanobacterial Adaptability
Revision of the Structure of Scytonemin Imine and Its Relationship to Scytonemin: Chromism and Implications for Astrobiology and Cyanobacterial Adaptability
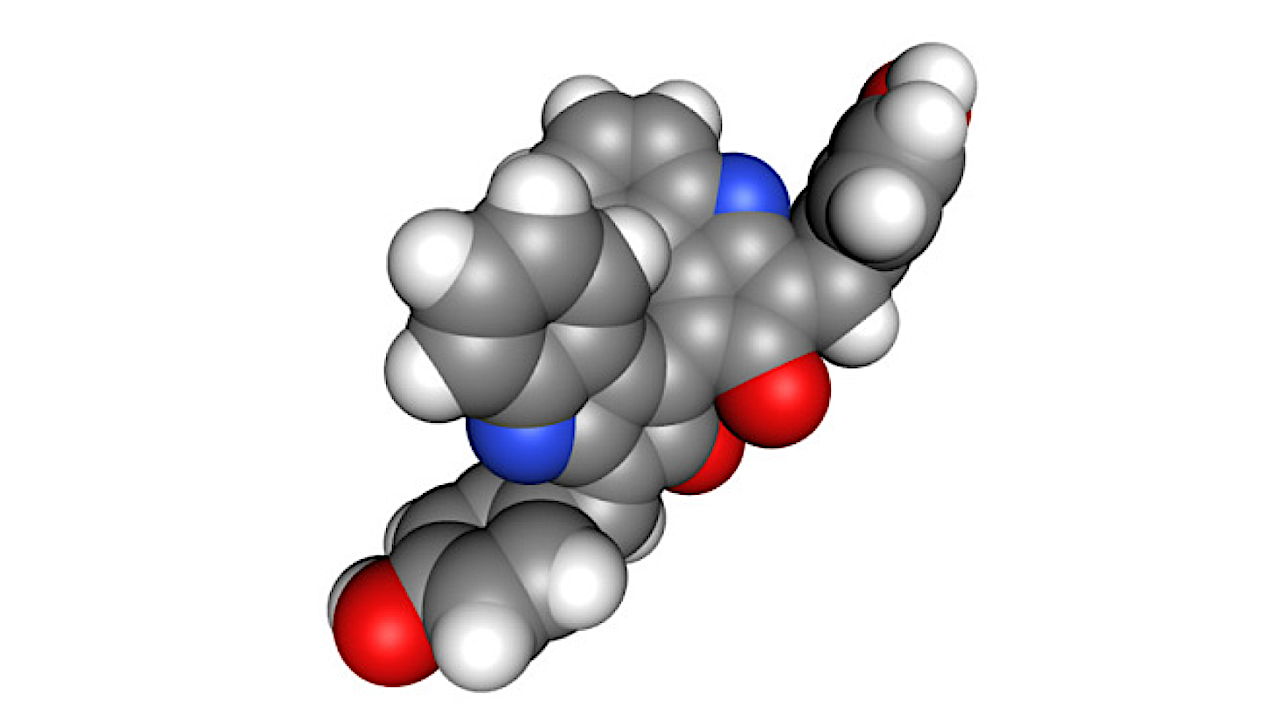

Scytonemin structure – Wikipedia
Scytonemin, a UV-protective pigment produced by cyanobacteria, is essential for microbial survival under extreme solar radiation.
Recent studies suggest its structural analog, scytonemin imine, may serve as a biosynthetic marker for cyanobacteria exposed to intense light. Here, we present a structural revision, revealing scytonemin imine as a cyclic hydropyrrolo[2,3-b] indole, rather than the previously proposed primary imine.
This reassignment is supported by 1D and 2D NMR, Q-TOF mass spectrometry, confirmatory synthesis, and DFT calculations. Our synthesis demonstrates that scytonemin converts to scytonemin imine under mild conditions-ammonia and acetone exposure-suggesting the imine adduct is likely an artifact of isolation.
However, our findings also indicate that this artifact may reveal a previously unrecognized in-vivo state of scytonemin, which is released upon condensation with acetone. This reactivity uncovers a new chromism within the scytonemin scaffold, supporting the idea that biogenic scytonemin analogs may filter visible light to regulate photosynthesis and protect against ROS-mediated photodamage during high light exposure or desiccation.
This chromatic transformation highlights scytonemin’s structural adaptability, offering insight into its role as a protective pigment in ancient and modern cyanobacteria and its relevance for understanding microbial adaptation to extreme environments.
Astrobiology
Stay Informed With the Latest & Most Important News
Previous Post
Next Post
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04True Anomaly hires former York Space executive as chief operating officer
04True Anomaly hires former York Space executive as chief operating officer -
 05Φsat-2 begins science phase for AI Earth images
05Φsat-2 begins science phase for AI Earth images -
 06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly