Now Reading: Supercooling, Glass Formation, and Mineral Assemblages upon Freezing of Salty Ice Grains from Enceladus’s Ocean
-
01
Supercooling, Glass Formation, and Mineral Assemblages upon Freezing of Salty Ice Grains from Enceladus’s Ocean
Supercooling, Glass Formation, and Mineral Assemblages upon Freezing of Salty Ice Grains from Enceladus’s Ocean


The analysis of micrometer-sized ice grains emitted into space by Saturn’s moon Enceladus suggests that the moon’s subsurface ocean may be habitable. However, the formation conditions of these ice grains are largely unknown.
Upon cooling, ocean droplets may supercool and then form a crystalline or glassy state, or a mixture of both. To investigate the processes of supercooling and glass formation in Enceladus’s ice grains, we performed differential scanning calorimetry experiments with Enceladus-relevant salt mixtures at cooling rates ranging from 5 K minute−1 to ∼1227 K minute−1 and extrapolated our results to faster cooling rates. We modeled the freezing of these solutions and associated mineral assemblages using the thermodynamic chemistry packages PHREEQC and Reaktoro.
Our results indicate supercooling of ∼25–30 K upon freezing from Enceladus’s saline ocean. Freshly formed ice grains should be predominantly crystalline but contain up to 5% glass. Fast cooling rates and high salt concentrations favor the formation of glasses, potentially enabling the preservation of organics and cells, if present. Salts in the grains crystallize in the following sequence: first phosphate, followed by carbonates, and then chlorides.
We find that the recently detected phosphates in Enceladus’s ice grains are likely Na2HPO4:12H2O. The pH values appear to vary among individual ice grains, depending on the stage of the freezing process, and these values may slightly differ from the pH of the moon’s bulk ocean.
Our experiments and models are relevant to other icy worlds with salty water reservoirs in their subsurfaces, such as Jupiter’s moon Europa or the dwarf planet Ceres.
Supercooling, Glass Formation, and Mineral Assemblages upon Freezing of Salty Ice Grains from Enceladus’s Ocean, The Planetary Science Journal (open access)
Astrobiology
Stay Informed With the Latest & Most Important News
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04True Anomaly hires former York Space executive as chief operating officer
04True Anomaly hires former York Space executive as chief operating officer -
 05Φsat-2 begins science phase for AI Earth images
05Φsat-2 begins science phase for AI Earth images -
 06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
06Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
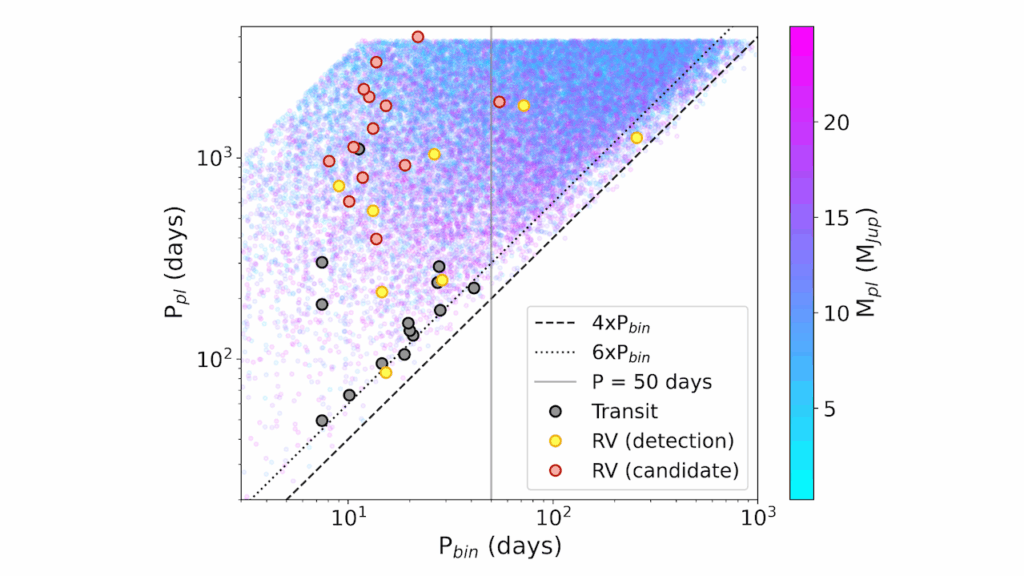
 07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly