Now Reading: Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors
-
01
Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors
Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors
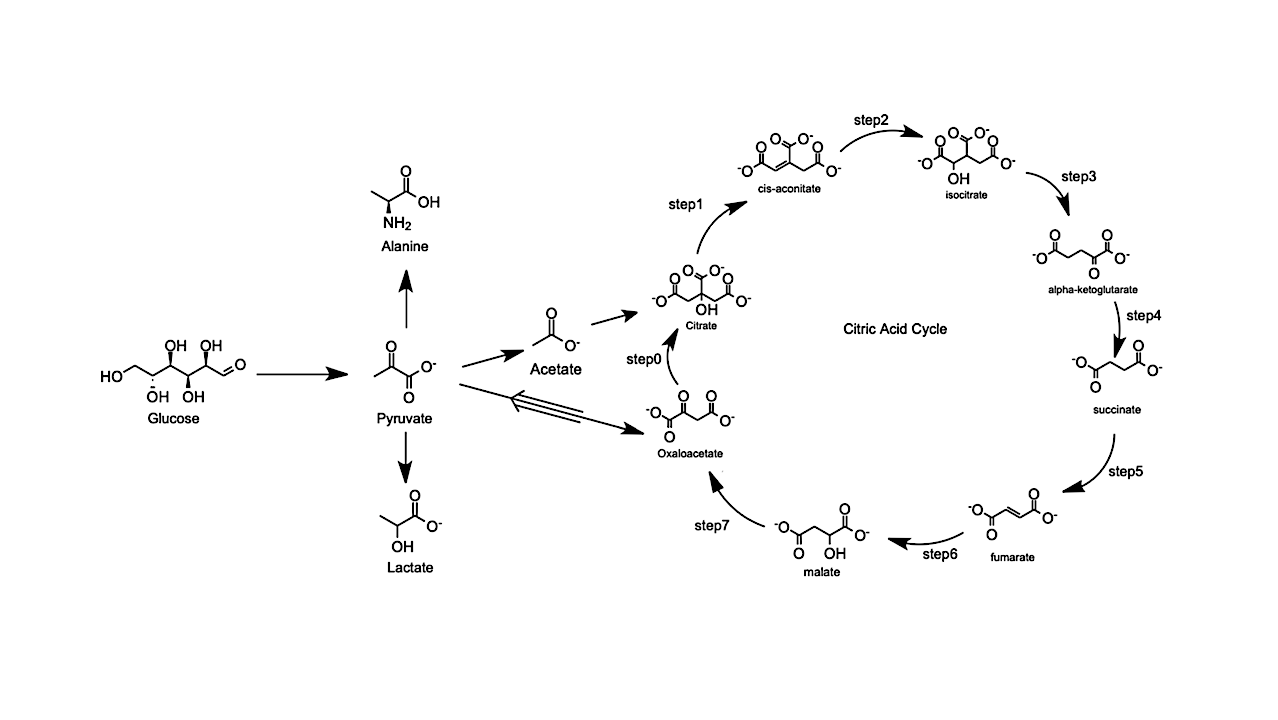

TCA cycle and the associated prebiotic network centered on pyruvate. The thin arrows denote biologically expected pathways in typical terrestrial environments for aerobic organisms, while the double arrow shows the computed spontaneous (exergonic) direction of the associated reaction in the majority of the ocean-world interior conditions considered in this study. –astro-ph.EP
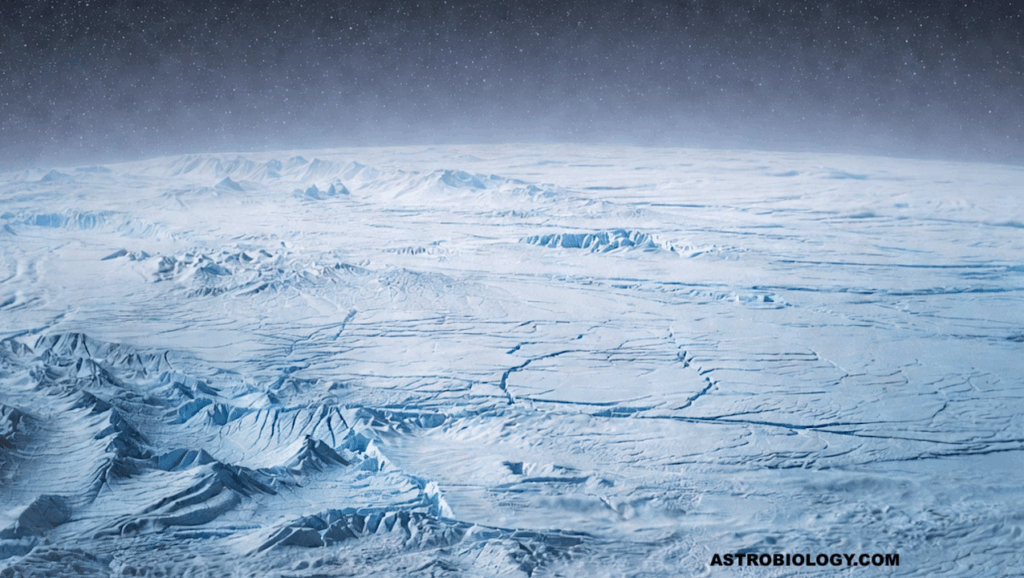
Icy ocean worlds attract significant interest for their astrobiological potential due to subsurface oceans, organics, and chemical energy sources.
We quantify thermodynamic viability of metabolism-relevant reactions at pressure-temperature conditions of Enceladus, Europa, Titan, Ganymede, and the Lost City Hydrothermal Field for comparison.
We examine the tricarboxylic acid (TCA) cycle and a plausible prebiotic reaction network leading to it, using DEWPython (based on the Deep Earth Water model) and SUPCRT to compute equilibrium constants and Gibbs free energy changes across temperatures from 0-1200°C and pressures between 1 bar-60 kbar.

Pipeline diagram for all the calculations and analysis throughout the paper. Colors denote various object classes, indicated in the legend. Relevant figures, tables, and method sections are also referenced. –astro-ph.EP
We found that across most oceanic P-T profiles, certain TCA cycle species accumulate (citrate, succinate) while others diminish (fumarate, oxaloacetate), suggesting ocean worlds’ conditions may not thermodynamically favor a unidirectional TCA cycle, requiring additional energy to overcome bottlenecks.
Similar bottlenecks exist at Lost City, which is inhabited. In the prebiotic network, pyruvate and acetate show remarkable stability, feeding the TCA cycle through citrate production, bypassing the oxaloacetate bottleneck. Formation of most TCA cycle species from inorganic compounds (CO2 + H2) is highly favored throughout ocean world geotherms, except for oxaloacetate.
While based on uncertain chemical concentrations, our non-equilibrium thermodynamic predictions are relatively insensitive to activity changes and may aid interpretation of future mission data. [ABRIDGED]

Affinity (left panels) and Gibbs free energy change (right panels) of the reactions involved in the prebiotic network. The curves show the PT curves of the studied moons and the depths near the Lost City Hydrothermal Field. The dashed curve indicates the critical affinity (A=0; left) or the transition between forced-endergonic and spontaneous-exergonic reaction states in equilibrium (right). — astro-ph.EP
Seda Işık, Mohit Melwani Daswani, Emre Işık, Jessica Weber, Nazlı Olgun Kıyak
Comments: 36 pages, 13 figures, accepted for publication in ACS Earth and Space Chemistry
Subjects: Earth and Planetary Astrophysics (astro-ph.EP)
Cite as: arXiv:2504.19369 [astro-ph.EP] (or arXiv:2504.19369v1 [astro-ph.EP] for this version)
https://doi.org/10.48550/arXiv.2504.19369
Focus to learn more
Submission history
From: Emre Işık
[v1] Sun, 27 Apr 2025 22:17:11 UTC (3,289 KB)
https://arxiv.org/abs/2504.19369
Astrobiology, Biochemistry,
Stay Informed With the Latest & Most Important News
-
 01Two Black Holes Observed Circling Each Other for the First Time
01Two Black Holes Observed Circling Each Other for the First Time -
 02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life
02From Polymerization-Enabled Folding and Assembly to Chemical Evolution: Key Processes for Emergence of Functional Polymers in the Origin of Life -
 03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series)
03Astronomy 101: From the Sun and Moon to Wormholes and Warp Drive, Key Theories, Discoveries, and Facts about the Universe (The Adams 101 Series) -
 04Φsat-2 begins science phase for AI Earth images
04Φsat-2 begins science phase for AI Earth images -
 05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters
05Hurricane forecasters are losing 3 key satellites ahead of peak storm season − a meteorologist explains why it matters -
 06Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors
06Thermodynamic Constraints On The Citric Acid Cycle And Related Reactions In Ocean World Interiors -
 07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly
07Binary star systems are complex astronomical objects − a new AI approach could pin down their properties quickly